Augest 12, 2024
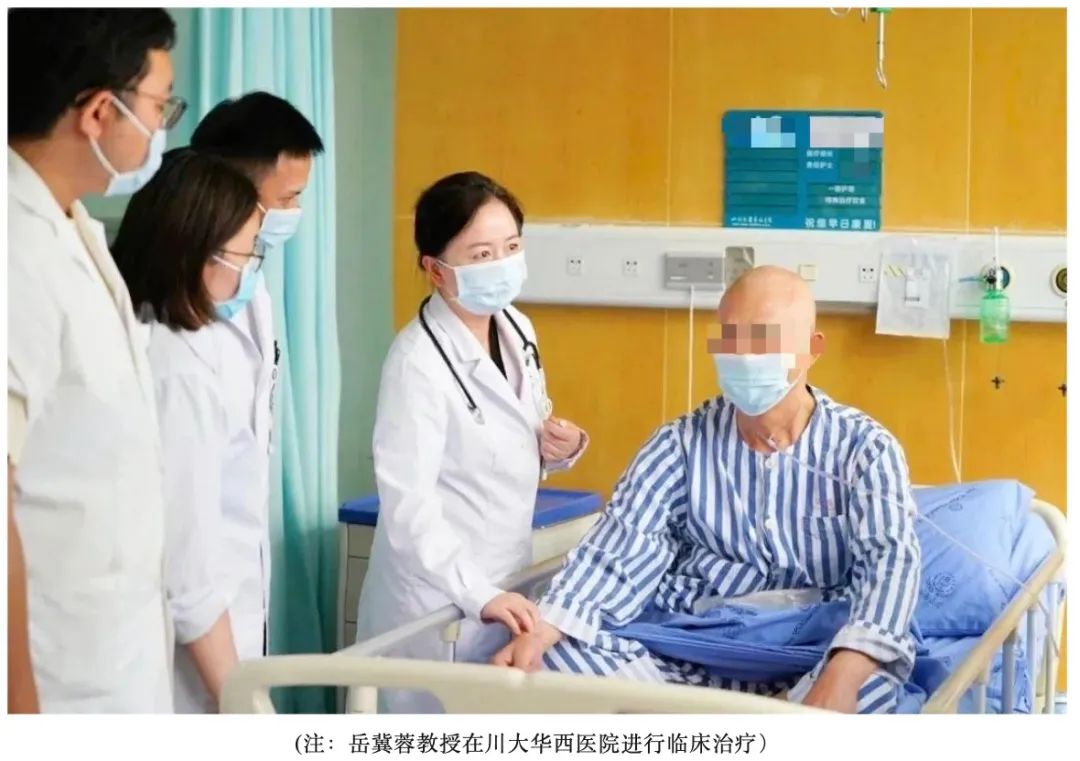
Recently, a groundbreaking global clinical trial for an rAAV gene therapy targeting sarcopenia has been initiated at West China Hospital, Sichuan University. Titled "A clinical study to evaluate the safety and efficacy of ZS112 injection in the treatment of sarcopenia patients," this trial marks a significant advancement in medical research. The successful treatment of the first patient sets the stage for potential transformative outcomes in the field of sarcopenia therapy.
The project was launched by a team of principal investigators, including Director Birong Dong and Director Jirong Yue from the National Clinical Research Center for Geriatric Diseases at West China Hospital, Sichuan University, as well as Director Chengqi He and Associate Director Qian Wang from the Rehabilitation Center. The drug, ZS112 injection, was originally developed by Sichuan Real & Best Biotechnology Co., Ltd. The first subject underwent gene therapy and completed the initial follow-up. At present, the subject is in good condition, and the therapeutic gene is correctly expressed, demonstrating the safety and efficacy of the ZS112 drug. This marks a critical transition of the ZS112 project from basic laboratory research results to clinical application, bringing new hope to patients with sarcopenia.
「至善唯新」自主研发的全球首个肌少症rAAV基因药物临床研究在四川大学华西医院启动November 13, 2023
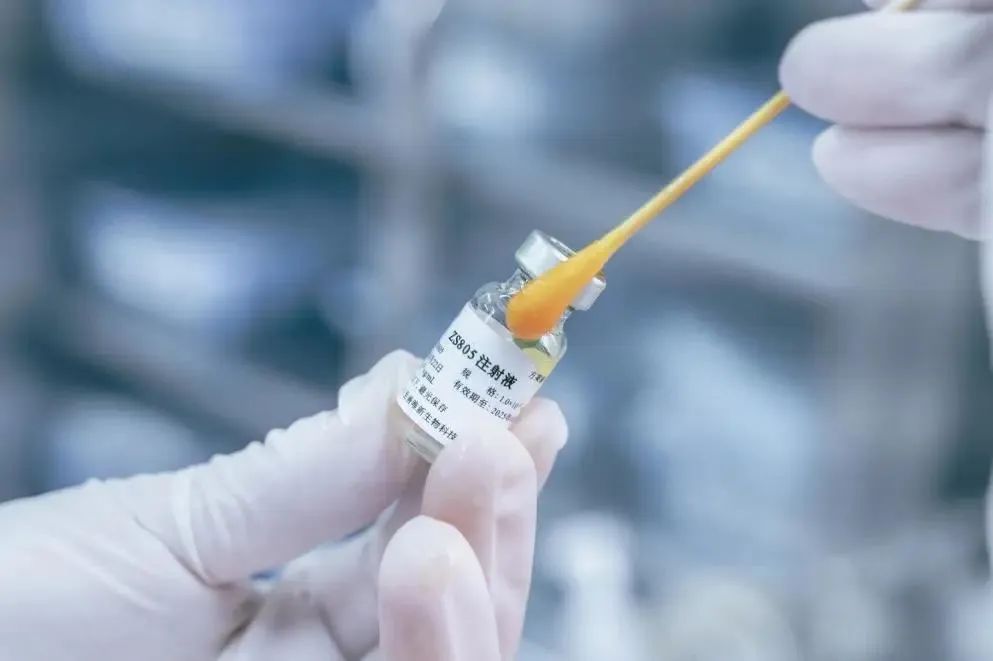
As of January 31, 2024, the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) has announced that Sichuan Real&Best Biotechnology Co., Ltd.'s groundbreaking Fabry disease gene therapy product ZS805 injection has received implied approval for clinical trials and is about to embark on Phase I/II clinical trials. This marks a significant milestone as Real&Best becomes the first domestic pharmaceutical company to gain clinical approval for an original gene therapy drug for Fabry disease.
国内首个!至善唯新rAAV基因药物临床研究启动,有望实现“一针治疗”! (qq.com)创新捷报丨至善唯新在法布雷病基因治疗领域再获突破 (qq.com)
June 28, 2023
On June 28, 2023, the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) announced that Sichuan Real&Best Biotechnology Co., Ltd. (referred to as 'Real&Best') has obtained implied approval for clinical trials for the Type 1 innovative drug 'ZS802 Injection.' The company is poised to initiate Phase I/II clinical trials. Following the clinical approval obtained in August 2022 for the initial project targeting Hemophilia B, Real&Best has achieved the distinction of being the first pharmaceutical company in China to secure clinical approval for original gene therapies for both Hemophilia A and B, thereby offering a more comprehensive treatment approach to address the cure for hemophilia.
创新捷报|至善唯新成为中国首家A、B两型血友病基因新药临床双证药企,血友病基因治疗领域再获突破 (qq.com)February 01, 2023
Established in 2018, Real&Best is dedicated to pioneering original gene therapy drugs using recombinant adeno-associated virus vectors (rAAV) to combat diseases in areas such as hematology, the central nervous system, and metabolism. Since its inception, Real&Best has successfully secured multiple rounds of financing, including a substantial Series A funding in the hundreds of millions and a recent Series A+ financing round that raised over 200 million RMB. Notable investors in Real&Best include Zhengxin Valley Capital, National Venture Capital, CV Capital, Puxin Capital, Delian Capital, Junshi Biosciences, Le Méridien, Anxin Guosheng Fund, Sichuan Talent Fund, among others.
【首发】至善唯新完成逾2亿元人民币A+轮融资,加速推进遗传病管线,布局代谢及衰老领域 (qq.com)Recent news
Augest 12, 2024
November 13, 2023
June 28, 2023
February 01, 2023