Given the considerable time and effort companies have invested in gene therapy, most prefer to play it safe when it comes to manufacturing. Accordingly, the large majority of recombinant adeno-associated virus (rAAV)-based therapies—which account for most gene therapies—are still produced with a method developed more than two decades ago, known as the ‘triple transfection’ technique. One of the central challenges of rAAV production is the need for a ‘helper virus’ to facilitate replication within a host mammalian cell. In the early days of rAAV, cultured cells would first be infected with adenovirus, after which they would be transfected with two DNA plasmids: one containing a pair of essential rAAV genes, and the other a sequence to be employed for the gene therapy in question. This approach yields a reasonable output of fully-assembled, gene therapy-ready rAAV particles. However, these must then be separated from the also-abundant adenovirus particles that contaminate the resulting virus preparation, and can put patients at risk. In 1998, two different research groups—one led by R. Jude Samulski at the University of North Carolina and the other by Peter Colosi at Avigen—determined that they could avoid these hassles by shifting key functions of the adenovirus on to a third, ‘helper’ plasmid. In this approach, researchers can simply transfect their preferred host cell—in most cases, human embryonic kidney (HEK) 293 cells—with all three plasmids in a stepwise procedure (see Figure 1). The combined activity of the adenovirus-derived genes on the helper plasmid with the rAAV genes on the second plasmid proved sufficient to generate functional rAAV particles containing the transgene from the third plasmid.
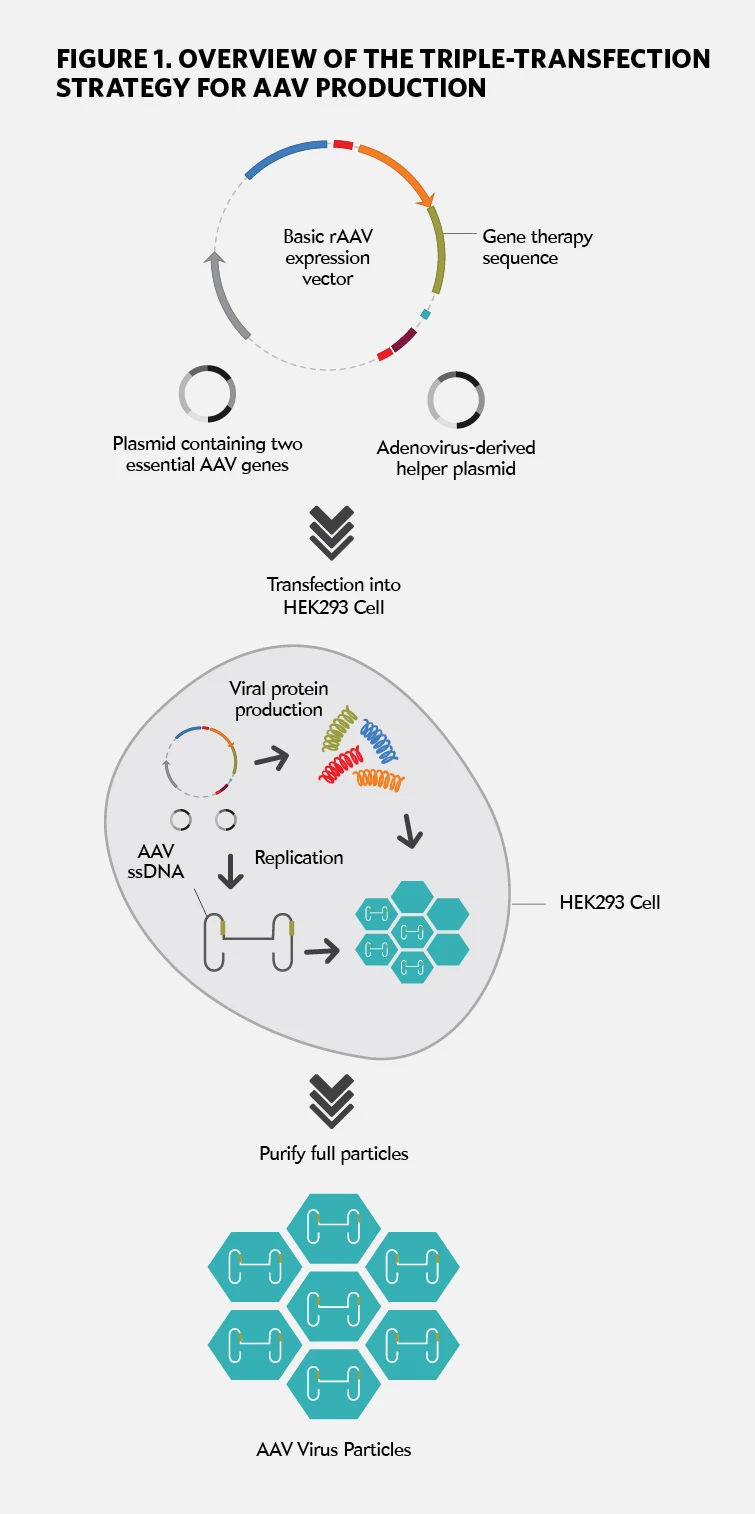
This approach eliminates the need for a ‘helper’ adenovirus by introducing key genes in a DNA plasmid (top), accompanied by two other plasmids. The three plasmids are transfected into the production cell line (middle), which produces mature AAV capsids containing the transgene of interest. Once purified (bottom), these viral particles can infect patient cells to deliver their DNA payload, but cannot replicate further.
